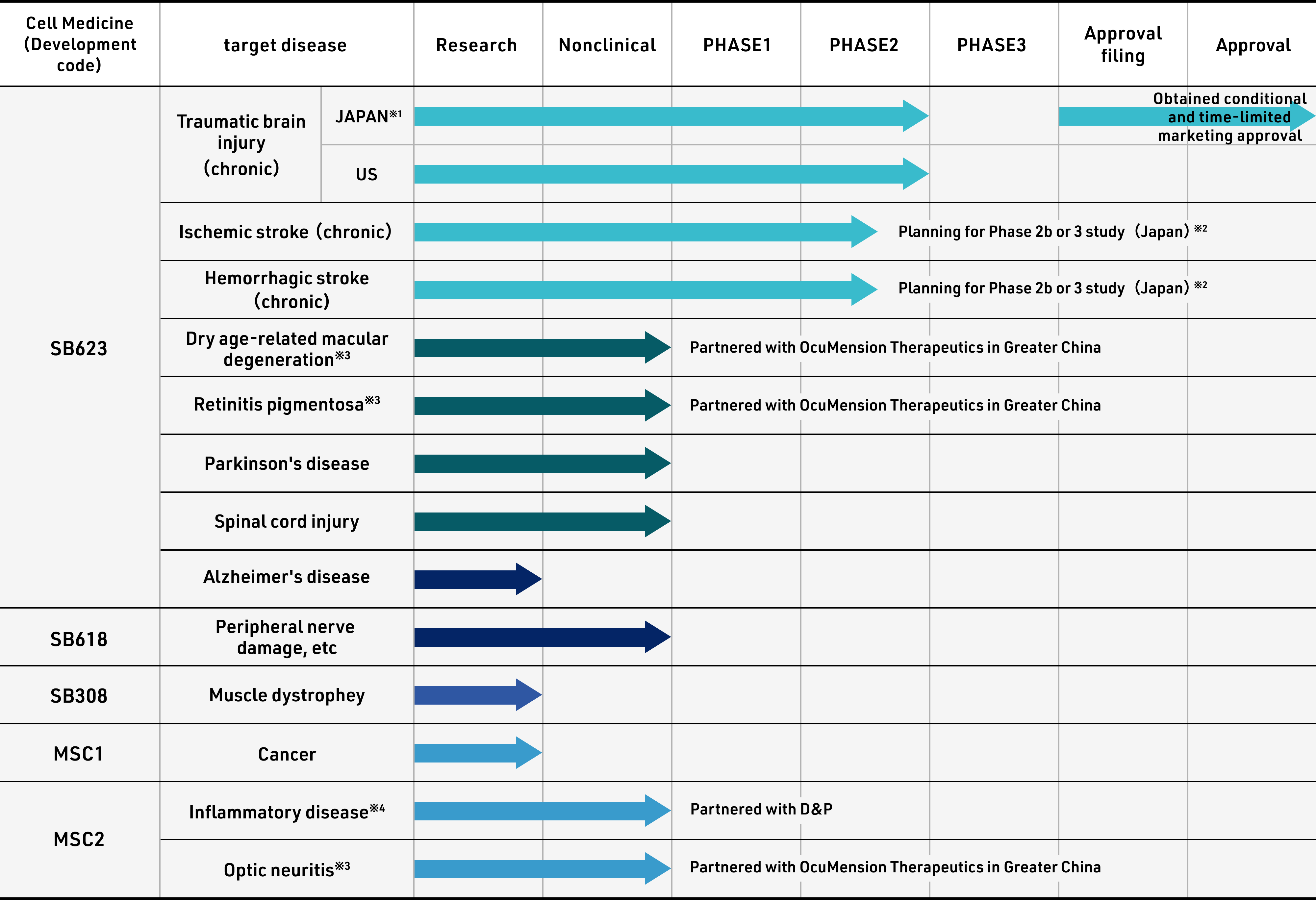
Status of Product Development
Progress with regenerative cell medicines currently in development
※1for the indication of improving chronic motor paralysis associated with traumatic brain injury.
※2Clinical trials will begin from Phase 2b as safety has been confirmed in previous clinical trials for ischemic stroke andTBI programs.
※3Joint development with OcuMension (Hong Kong) Limited.
※4Formed a business partnership with D&P Bioinnovations, Inc. for the development and commercialization of regenerative esophageal implant.
Expanded Access Program